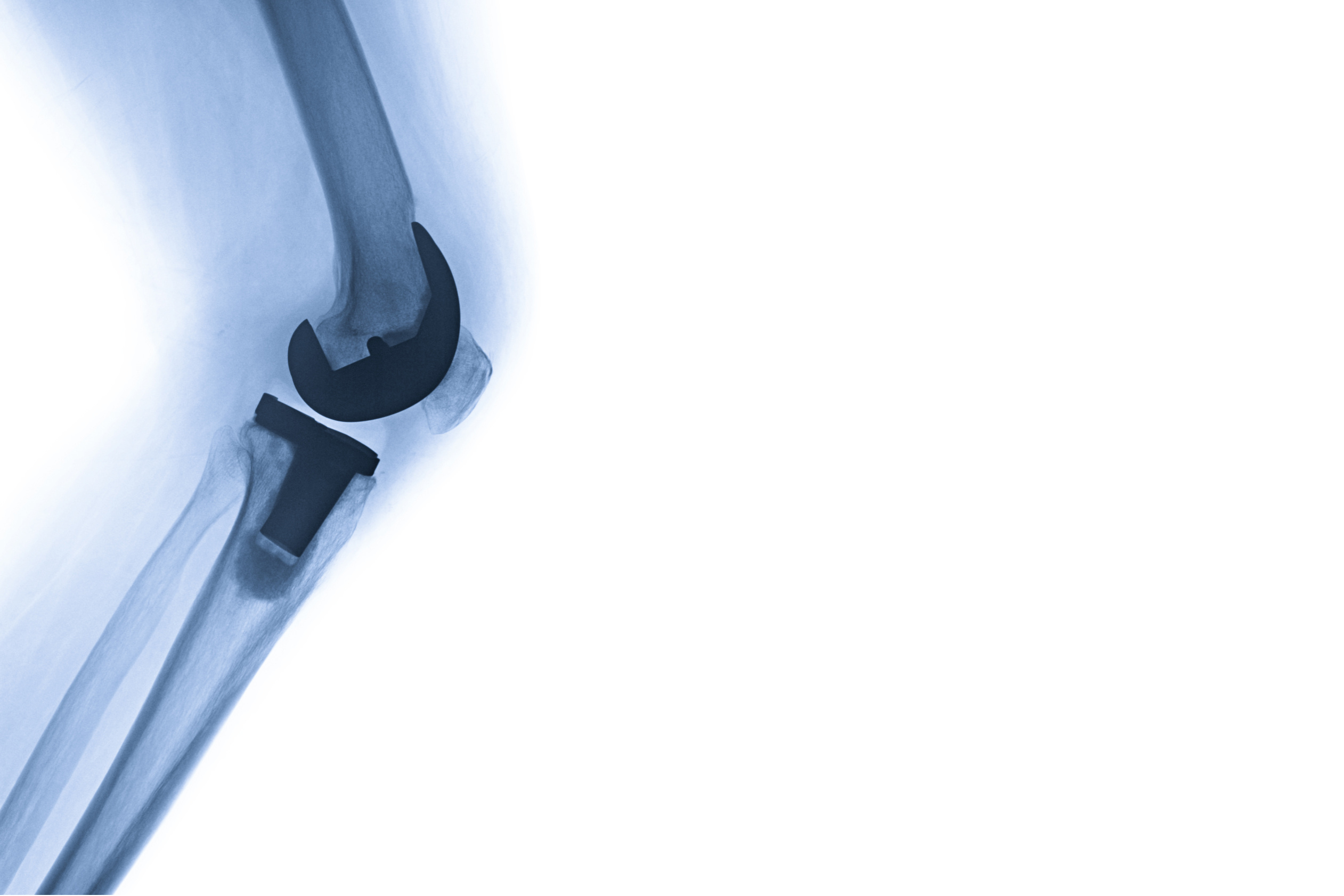
Exactech is facing a lawsuit after it issued a recall for its Optetrak artificial knee implants, according to an article published in KFF Health News. The lawsuit will involve over 1,100 patients who received defective implants and claimed that they have to undergo risky revision surgery to replace the parts. One such patient involved in the lawsuit, 71-year-old Ron Irby, said that he expected his Optetrak knee replacement to last longer than 20 years. After 3 years, the implant had become worn out and needed to be replaced. According to Exactech—which denied the allegations brought forward in the lawsuit—a packaging error may have resulted in premature deterioration of the plastic in up to 140,000 knee implants dating back to 2004. However, whistleblowers and surgeons have revealed that the company ignored warnings regarding the lack of durability, high fragility, and premature loosening of the components of its implants in as little as 2 to 3 years, as well as failed to report the defects to the U.S. Food and Drug Administration’s MAUDE databank. Instead of issuing a recall, the company has been accused of attempting to replace the problematic components of the implants in a “silent recall” which was issued in 2022. Experts stressed that the poor quality of the joints and lack of transparency from Exactech may have not only caused patients pain and limited joint functionality but may have also exacted an emotional toll as a result of the need for additional procedures. Further legal records indicate that the company may have aimed to increase profits by falsely advertising product longevity; utilizing inferior manufacturing practices; downplaying, concealing, and neglecting to address the failures of its knee replacements; and only taking action if contacted by regulatory authorities—at the cost of patient safety.