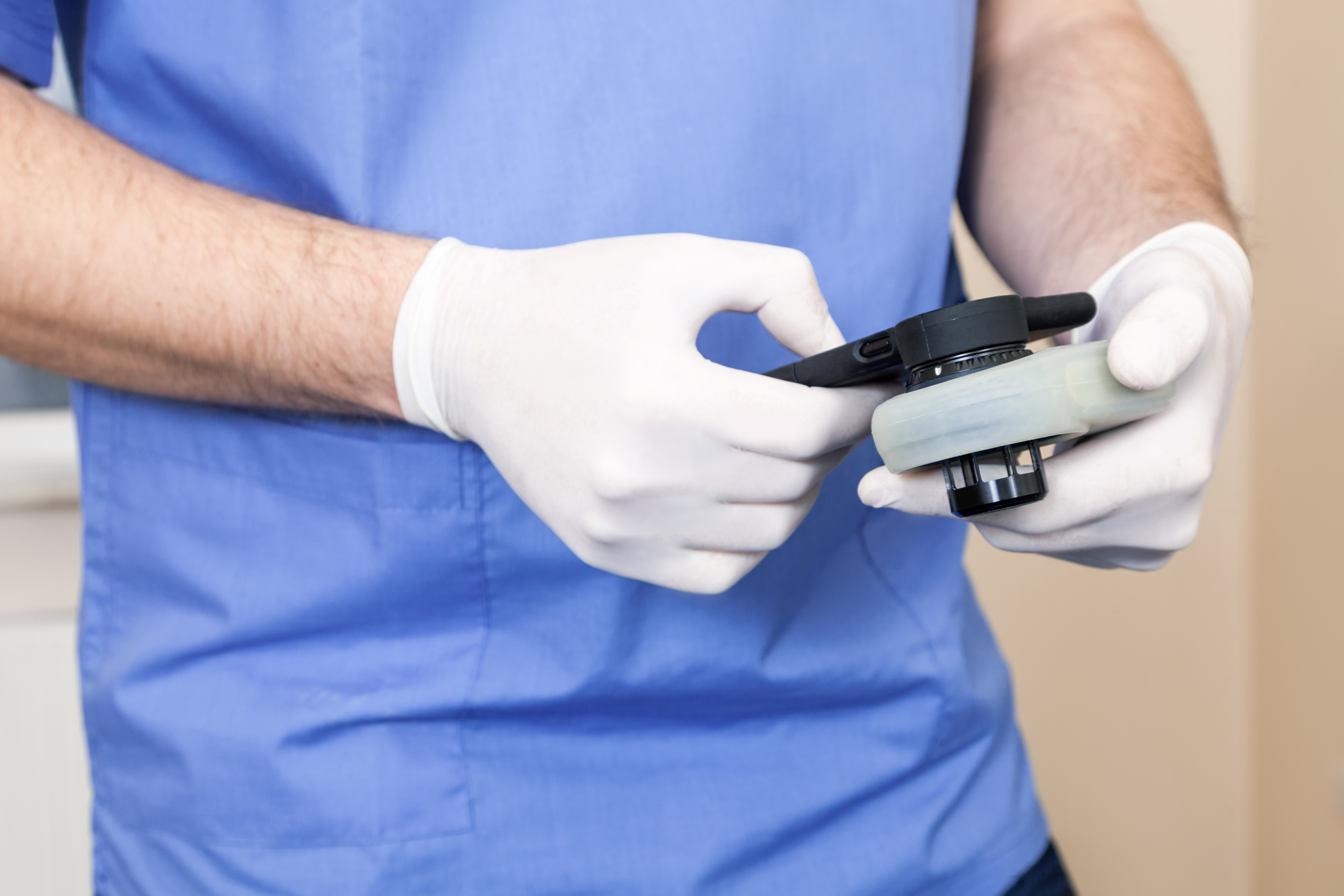
The medical technology company DermaSensor’s artificial intelligence (AI)-based handheld device has been cleared by the U.S. Food and Drug Administration (FDA) for the detection of skin cancer, according to an article published in Forbes. The novel device works by taking spectral recordings of patients’ skin lesions and utilizing AI to make an immediate assessment on the basis of three types of skin cancers: basal cell carcinoma, squamous cell carcinoma, and melanoma. The FDA indicated that the device should only be used on lesions determined to be suspicious and that DermaSensor will be required to validate the device’s performance in additional clinical trials involving diverse demographic groups. Early data suggested that the novel device had a sensitivity of 96% across all 224 types of skin cancer and reduced missed diagnoses from 18% to 9%.