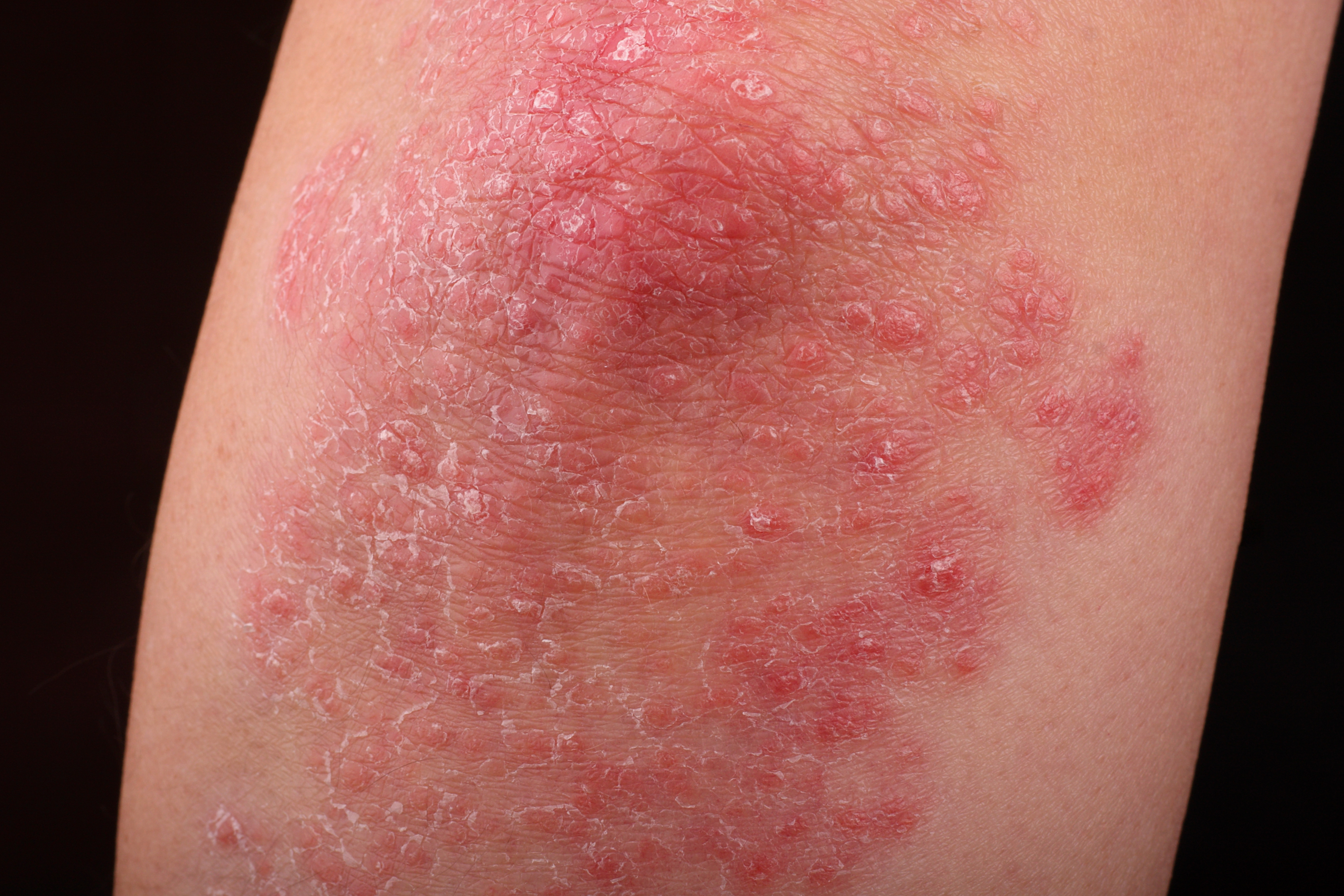
The novel oral interleukin 23–receptor agonist peptide JNJ-77242113 showed greater efficacy than placebo in patients with plaque psoriasis, according to a recent study published by Bissonnette et al in The New England Journal of Medicine. In a phase II dose-finding trial, researchers randomly assigned 255 patients with moderate-to-severe plaque psoriasis to receive either JNJ-77242113 at doses of 25 mg once daily, 25 mg twice daily, 50 mg once daily, 100 mg once daily, or 100 mg twice daily, or placebo. The primary endpoint of the trial was at least a 75% reduction from baseline in Psoriasis Area and Severity Index score. After a follow-up of 16 weeks, the researchers found that 37%, 51%, 58%, 65%, and 79% of the patients who received the respective JNJ-77242113 doses experienced 75% reductions in their Psoriasis Area and Severity Index scores, compared with 9% of those who received placebo. They reported that the patients who received JNJ-77242113 and placebo experienced similar incidences of adverse events such as COVID-19 infections and nasopharyngitis; however, there were no dose-related increases in adverse events among patients who received JNJ-77242113. The researchers hope the novel agent can provide a new treatment option for patients with plaque psoriasis.